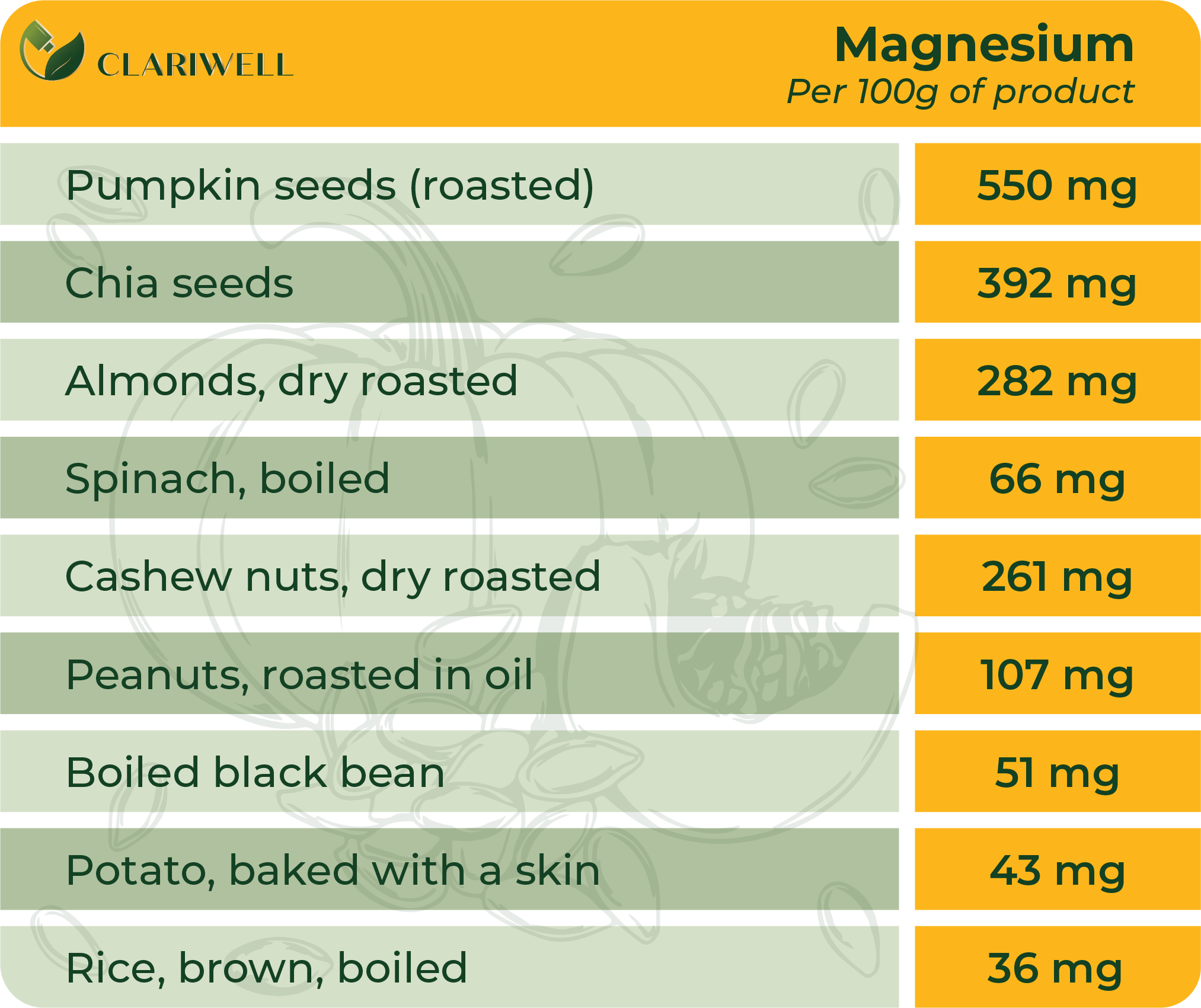
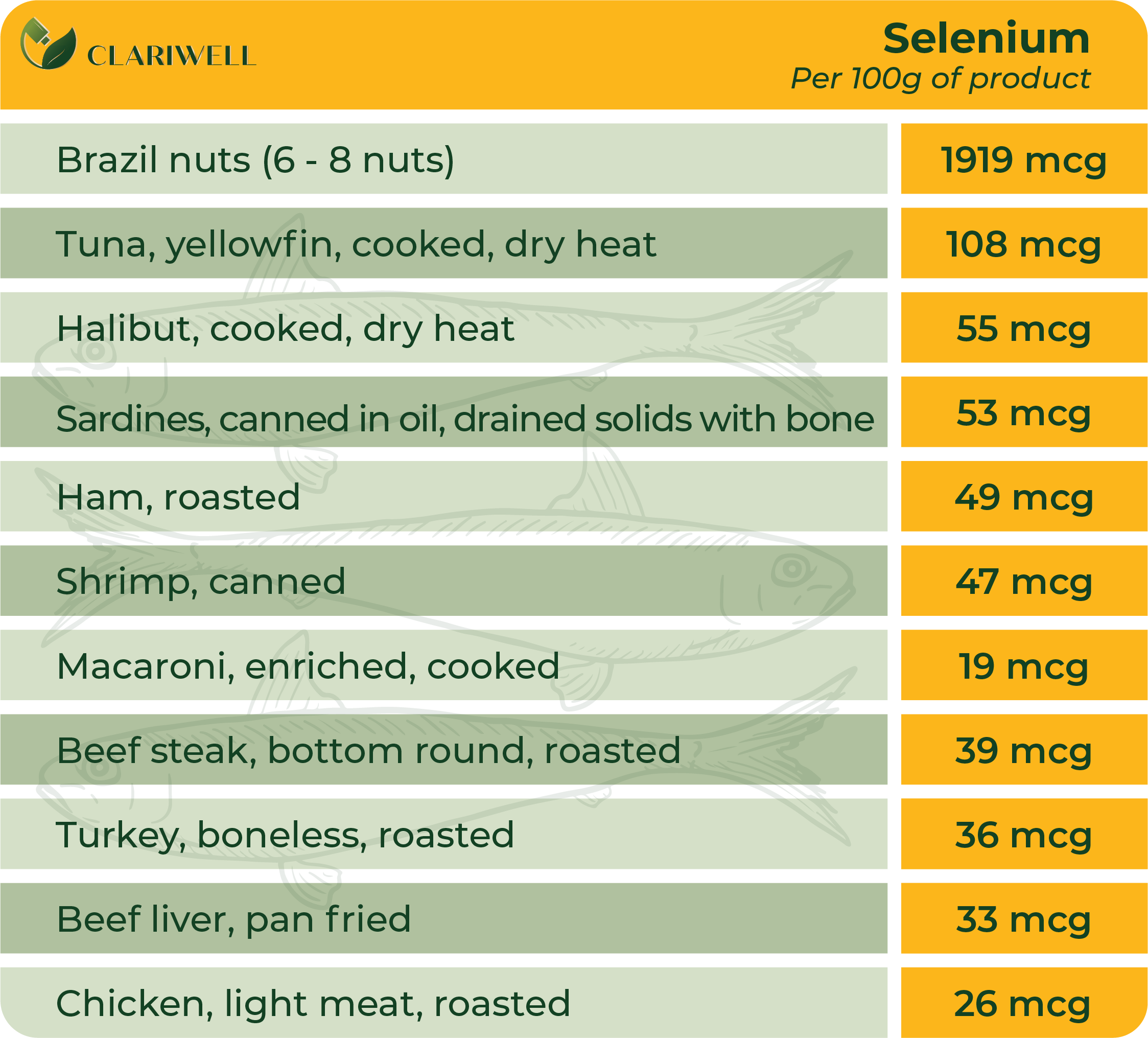
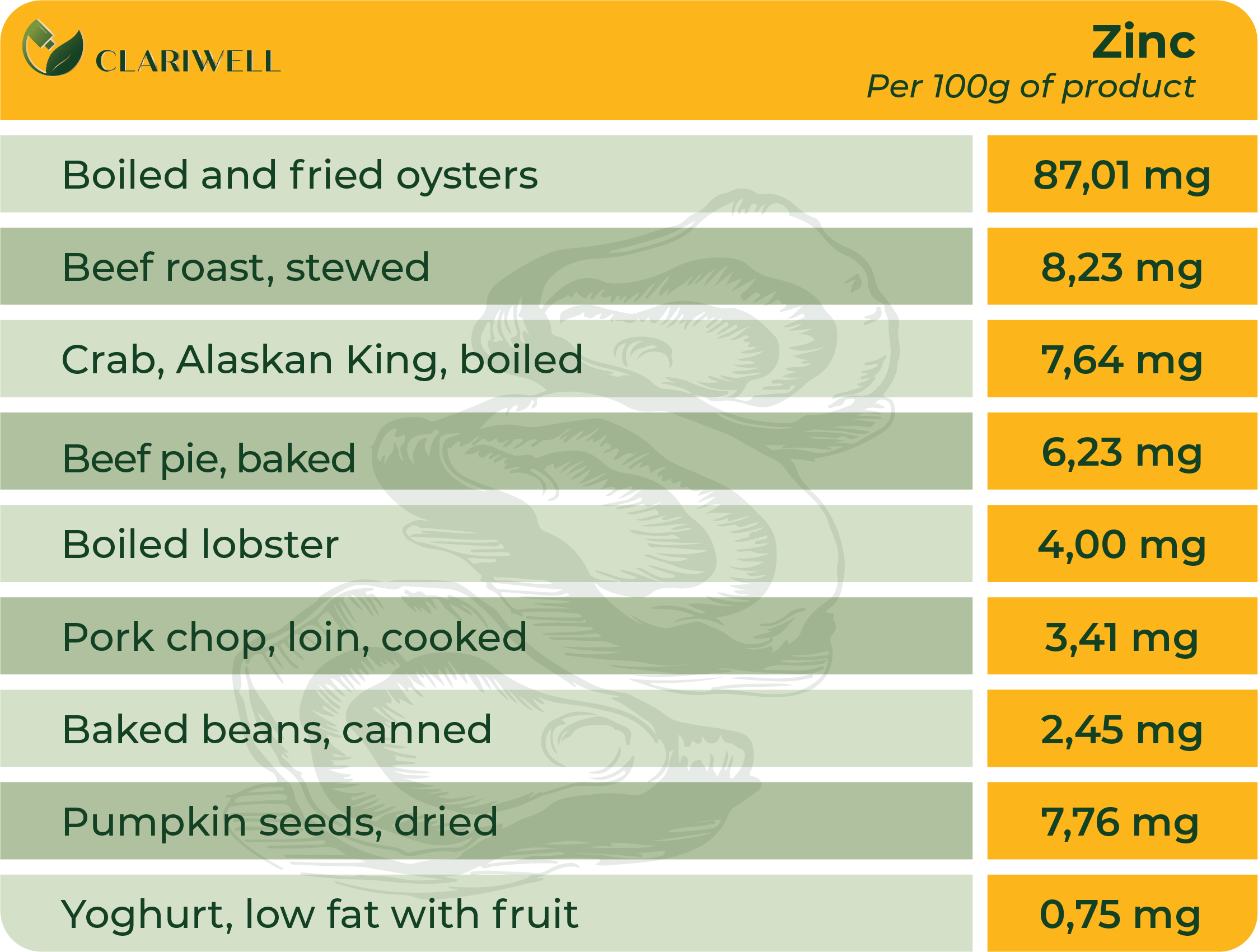
Magnesium
Magnesium is the fourth most frequently occurring mineral in a human body. It plays a vital role in normal functioning and division of cells, energy metabolism, normal functioning of nerves and muscles, maintaining cardiac rhythm and immunity support. Magnesium in sufficient doses is believed to have a favourable impact on migraine, chronic pain, anxiety and depression.
Magnesium is a natural mineral that was discovered in 1755. It is the eighth most abundant element on earth and occurs naturally only in combination with other elements. In nature, it is found in large mineral deposits such as magnesite and dolomite rocks.
Magnesium is required by entire body as it enables muscle contraction, conductivity of nerves. Magnesium keeps a steady heartbeat and strong immune system.
Magnesium is an essential nutrient that is involved in many key metabolic reactions such as energy production, glycolysis, and the synthesis of nucleic acids and proteins.
Magnesium also plays a role in the active transport of calcium and potassium ions across cell membranes, a process that is important to conductivity of nerves, muscle contraction, and normal heart rhythm.
From a neurological standpoint, magnesium plays an essential role in nerve transmission and neuromuscular conduction. It also has a protective role against excessive excitation that can lead to destruction of neurons (excitotoxicity) and has been implicated in multiple neurological disorders. Due to these important functions within the nervous system, Magnesium is a mineral of intense interest for the potential prevention and treatment of neurological disorders. Current scientific literature reviews the applicability of Magnesium for migraine, chronic pain, epilepsy, Alzheimer’s, Parkinson’s, and stroke, as well as the commonly comorbid conditions of anxiety and depression.
Magnesium is very important for our body but not all people have it in sufficient quantities. Why? The reasons can be quite different. U.S. National Institute of Health lists the following Groups at Risk of Magnesium Inadequacy:
People with gastrointestinal diseases
The chronic diarrhea and fat malabsorption resulting from Crohn’s disease, gluten-sensitive enteropathy (celiac disease), and regional enteritis can lead to magnesium depletion over time. Resection of the small intestine, especially the ileum, typically leads to malabsorption and magnesium loss.
People with type 2 diabetes
Magnesium deficits and increased urinary Magnesium excretion can occur in people with insulin resistance and/or type 2 diabetes. The Magnesium loss appears to be secondary to higher concentrations of glucose in the kidney that increase urine output.
People with alcohol dependence
Magnesium deficiency is common in people with chronic alcoholism. In these individuals, poor dietary intake and nutritional status; gastrointestinal problems, including vomiting, diarrhea, and steatorrhea (fatty stools) resulting from pancreatitis; renal dysfunction with excess excretion of Magnesium into the urine; phosphate depletion; vitamin D deficiency; acute alcoholic ketoacidosis; hyperaldosteronism secondary to liver disease can all contribute to decreased Magnesium status.
Several types of medications have a potential to interact with Magnesium supplements or affect Magnesium status. A few examples are provided below. People taking these and other medications of these groups on a regular basis should discuss Magnesium intakes with their healthcare providers.
Bisphosphonates
Magnesium-rich supplements or medications can decrease the absorption of oral bisphosphonates, such as alendronate (Fosamax®), used to treat osteoporosis. Magnesium-rich supplements or medications and oral bisphosphonates should be taken at least 2 hours from each other.
Antibiotics
Magnesium can form insoluble complexes with tetracyclines, such as demeclocycline (Declomycin®) and doxycycline (Vibramycin®), as well as quinolone antibiotics, such as ciprofloxacin (Cipro®) and levofloxacin (Levaquin®). These antibiotics should be taken at least 2 hours before or 4–6 hours after a Magnesium-containing supplement.
Diuretics
Chronic treatment with loop diuretics, such as furosemide (Lasix®) and bumetanide (Bumex®), and thiazide diuretics, such as hydrochlorothiazide (Aquazide H®) and ethacrynic acid (Edecrin®), can increase the loss of Magnesium in urine and lead to Magnesium depletion. In contrast, Potassium-sparing diuretics, such as amiloride (Midamor®) and spironolactone (Aldactone®), reduce Magnesium excretion.
Magnesium is possibly one of the most studied substances for people suffering from a migraine or headache. Some studies show that migraine sufferers tend to have lower magnesium levels than those who do not have this problem. Some scientists believe magnesium blocks signals in the brain that lead to migraine with aura or changes in vision and other senses. Studies also reveal that magnesium inhibits certain pain-inducing chemicals. Besides, it seems that lowering of magnesium levels also leads to narrowing of brain blood vessels potentially contributing to migraine.
Migraine’s neurological disorder is characterized by having pain in head and other various symptoms such as nausea, emesis, photophobia, phonophobia, and sometimes visual sensory disorders. Magnesium is a necessary ion for human body and has a crucial role in health and life maintenance. One of the main roles of Magnesium is to conserve neurons electric potential. Therefore, magnesium deficiency can cause neurological complications. Migraine is usually related to low amounts of Magnesium in serum and cerebrospinal fluid. Deficits in magnesium have significant role in the pathogenesis of migraine. Magnesium has been extensively used in migraine prophylaxis and treatment .
In scientific trials participants received daily magnesium supplementation for migraine prevention at varying doses (between 400mg and 1,200mg a day) for a varying period therefore it is difficult to define precisely the amount of magnesium a person needs to prevent migraine and it, most probably, is subject to individual factors (depending on the severity of magnesium deficit in that person).
However, some organisations, like Canadian Headache Association, recommend preventative magnesium therapy for adults and particularly a special dose of elementary magnesium of 600mg per day.
If you notice that magnesium-containing supplements do not alleviate your migraine attacks, it could be due to two underlying causes:
- Food supplement that you are using has poor bioavailability, meaning that it is not well absorbed. Usually, it is the case with poor quality food supplements which have low value of elemental magnesium (amount of magnesium indicated in a food supplement does not always equal the amount of elemental magnesium in the product) as well as different forms of magnesium has better or worse bioavailability.
- If diarrhoea appears after taking the daily dose, the food supplement is not absorbed good enough and does not give desirable effect.
Although there is no universally accepted definition, stress can be explained as a complex adaptive biochemical, physiological, psychological and gene expression change in the body (stress response) caused by a stimulus (stressor) and interpreted by the brain as dangerous.
The level of magnesium content in the body is closely related to the level of stress, since both stress and hypomagnesaemia (reduced magnesium in the blood) increase the negative effects of each other. In the case of hypomagnesaemia, as a result of stress, a series of disorders develop, for example, light-sensitive headaches, fibromyalgia (disorders described by widespread muscle and skeletal system pain accompanied by fatigue, sleep, memory disorders, etc.), chronic fatigue syndrome, audiogenic stress, cold stress and physical stress.
The transfer of magnesium from the intracellular (inside the cell) to the extracellular space primarily provides a protective role to mitigate the adverse effects of stress, but prolonged periods of stress lead to progressive magnesium deficiency and adverse health consequences.
A growing number of studies confirm that psychological stress promotes oxidative stress, mainly due to the autooxidation of catecholamines, and psychological stress increases lipid peroxidation, increases markers of DNA oxidative damage, and decreases plasma antioxidant activity. It is important here that magnesium antagonises (counteracts) many of these processes
- The word "magnesium" comes from the name of the Greek region Magnesia, where compounds of this element occur naturally.
- Milk of Magnesia, which works as a laxative and to treat indigestion, is a compound of magnesium, hydrogen and oxygen molecules.
- Don't put out a magnesium fire with water. After spraying the burning magnesium with water, it will start burning even faster, with a sharp flame.
- Magnesium ions have sour and bitter taste. A small amount of Magnesium gives taste to the mineral water.
- Magnesium is the 11th most abundant element in the human body by mass. Magnesium ions are found in every cell of the body.
- About 60% of the Magnesium in the human body is found in the skeleton, 39% in the muscle tissue, and 1% is extracellular.

Selenium
Among many others, selenium is one of the most important microelements required for normal functioning of human organs. This element plays important role in metabolism, thyroid function and protection of cells from damage caused by oxidative stress. Selenium is also an immunity booster, it improves brain function and reduces the risk of cardiovascular diseases.
Selenium was discovered in 1817 in Gripsholm, a Swedish city, by a Swedish chemist Jacob Berzelius. Selenium is a commonly occurring element in nature. It can be found in the atmosphere, lithosphere, biosphere, and hydrosphere of the Earth. The amount of this element present in nature and in the human organism is very diverse depending on the geographic region and diet.
The total amount of selenium in a human organism is ~3–20 mg.
Selenium is a trace element that is naturally present in many foods, added to others, and available as a dietary supplement. Selenium, which is nutritionally essential for humans, is a constituent of more than two dozen selenoproteins that play critical roles in reproduction, thyroid hormone metabolism, DNA synthesis, and protection from oxidative damage and infection.
Selenium is incorporated into selenoproteins that have a wide range of pleiotropic effects, ranging from anti oxidant and anti-inflammatory effects to the production of active thyroid hormone. In the past 10 years, the discovery of disease associated polymorphisms in seleno protein genes has drawn attention to the relevance of selenoproteins to health. The essential biological importance of selenium is associated with its occurrence in proteins and enzymes. Several selenium-dependent enzymes in which the active center contains selenium in the form of selenocysteine moiety have been identified. The best-characterized selenoenzymes commonly occurring in mammals are glutathione peroxidase, selenoprotein P, and thyroxine 5-deiodinase. Glutathione peroxidase and selenoprotein P catalyze redox reactions. Other enzymatic proteins that are involved in important functions of the organisms are formate dehydrogenase, nicotinic acid hydroxylase, glycine reductase, thiolase, and xanthine dehydrogenase.
Low selenium status has been associated with increased risk of mortality, poor immune function, and cognitive decline.
Higher selenium status or selenium supplementation has antiviral effects, is essential for successful male and female reproduction, and reduces the risk of autoimmune thyroid disease. Prospective studies have generally shown some benefit of higher selenium status on the risk of prostate, lung, colorectal, and bladder cancers, but findings from trials have been mixed, which probably emphasises the fact that supplementation will confer benefit only if intake of a nutrient is inadequate.
The eff ects of selenium on human health are multiple and complex, necessitating further research to optimize the benefits and reduce the risks of this potent trace mineral.
Prolonged selenium deficiency in human organism leads to serious diseases. Deficiency of this element adversely affects the functioning of the cardiovascular system and can be a direct cause of myocardial infarction. It is associated with endemic diseases: Keshan and Kashin-Beck.
As a result of epidemiological studies, it was concluded that moderate deficiency of selenium in daily diet affects the development of diseases resulting from reduced immunity. Selenium deficiency in daily diet can adversely affect the functioning of the nervous system. Among individuals with selenium deficiency, development of depression, or intensification of anxiety is observed; Alzheimer’s disease is also developed. This element is considered to be crucial in reducing the virulence of HIV and in decreasing the progression to full-blown AIDS. Selenium deficiency in pregnant women negatively affects the development of the embryo. Excess of selenium can be toxic to the organism. Acute selenium poisoning is rarely observed. The accurate determination of harmful doses of selenium is difficult because of the occurrence of various chemical forms of this element. A toxic effect on the organism can be exerted by both organic and inorganic forms of selenium. Toxicity of selenium (depends on the dose) is associated with competitive inhibition between selenium and sulfur, leading to the onset of sulfur metabolism (transformation). Selenium may substitute sulfur in amino acids (cysteine and methionine), whereas the inorganic compounds displace sulfur during the synthesis of mercapturic acids and during the reaction of selenites with thiol groups.
Groups at Risk of Selenium Inadequacy
Selenium deficiency is rare and selenium deficiency in isolation rarely causes overt illness. The following groups are among those most likely to have inadequate intakes of selenium.
1. People living in selenium-deficient regions
People in some countries whose diet consists primarily of vegetables grown in low-selenium areas are at risk of deficiency. The lowest selenium intakes in the world are in certain parts of China, where large proportions of the population have a primarily vegetarian diet and soil selenium levels are very low. Average selenium intakes are also low in some European countries, especially among populations consuming vegan diets. Although intakes in New Zealand were low in the past, they rose after the country increased its importation of high-selenium wheat.
2. People undergoing kidney dialysis
Selenium levels are significantly lower in patients undergoing long-term hemodialysis than in healthy individuals. Hemodialysis removes some selenium from the blood. In addition, hemodialysis patients are at risk of low dietary selenium intakes due to anorexia resulting from uremia and dietary restrictions. Although selenium supplementation increases blood levels in hemodialysis patients, more evidence is needed to determine whether supplements have beneficial clinical effects in these individuals.
3. People living with HIV
Selenium levels are often low in people living with HIV, possibly because of inadequate intakes (especially in developing countries), excessive losses due to diarrhea, and malabsorption. Observational studies have found an association between lower selenium concentrations in people with HIV and an increased risk of cardiomyopathy, death, and, in pregnant women, HIV transmission to offspring and early death of offspring.
Selenium in the diet. Adults who are not found or established to be deficient in selenium are normally required 55 mcg (micrograms) for men and for women, but for pregnant women 60 mcg of selenium per day
Selenium can interact with certain medications, and some medications can have an adverse effect on selenium levels. One example is provided below. Individuals taking this and other medications on a regular basis should discuss their selenium status with their healthcare providers.
Cisplatin
Cisplatin, an inorganic platinum chemotherapy agent, is used to treat ovarian, bladder, lung, and other cancers. Cisplatin can reduce selenium levels in hair and serum but whether these reductions have a clinically significant impact is not known. Some small studies have shown that selenium supplementation can reduce cisplatin’s toxicity but the authors of a Cochrane review concluded that the evidence that selenium supplementation alleviates the side effects of chemotherapy is insufficient.
Health Risks from Excessive Selenium
Chronically high intakes of the organic and inorganic forms of selenium have similar effects. Early indicators of excess intake are a garlic odor in the breath and a metallic taste in the mouth. The most common clinical signs of chronically high selenium intakes, or selenosis, are hair and nail loss or brittleness. Other symptoms include lesions of the skin and nervous system, nausea, diarrhea, skin rashes, mottled teeth, fatigue, irritability, and nervous system abnormalities.
There are a number of indications that selenium is important to the brain: during selenium depletion the brain receives a priority supply; the turnover rate of some neurotransmitters is altered in selenium deficiency; supplementation with selenium reduced intractable epileptic seizures in children; low plasma selenium concentrations in the elderly were significantly associated with senility and accelerated cognitive decline and brain selenium concentration in Alzheimer’s patients was only 60% of that in controls. Furthermore, the brain is deficient in catalase, thus peroxidation products such as hydrogen peroxide and primary peroxides must be removed by the antioxidant selenoenzymes.
A beneficial effect of selenium status on mood has been shown, at least when selenium status is “marginal”. In three studies, low selenium status was associated with a significantly greater incidence of depression and other negative mood states such as anxiety, confusion, and hostility.
Serum selenium concentrations decline with age. Marginal or deficient selenium concentrations might be associated with age-related declines in brain function, possibly due to decreases in selenium’s antioxidant activity.
Selenium, incorporated into specific seleno-enzymes, is essential to proper thyroid function and protect cells from oxidative damage induced by H2O2 during thyroid hormone synthesis while study has shown that oxidative stress markers are associated with cognitive decline in a highly cognitive functioning population.
Selenium-dependent glutathione reductase and selenoproteins are important for their antioxidant activity, which is vital for the protection of the organism. Selenium affects the metabolic pathways by changing the activity of selenoproteins and plays a role in cellular defense against oxidative stress. Selenium concentration regulates the expression of selenoproteins. Different selenium concentrations may affect immunity and energy metabolism diversely. Increased levels of stress biomarkers have been reported in depression in recent studies, and this suggests that oxidative stress may be an important factor in the pathogenesis of depression. Selenium may have a protective role against anxiety and depression, possibly due to its protective effect on oxidative stress.
Lastly, selenium could potentially exert antidepressant effects through its modulatory role in various neurotransmitter systems. Selenium has been found to have significant modulatory effects on the dopaminergic, serotonergic, and noradrenergic systems, which are all involved in the physiopathology of depression and other psychiatric illnesses.
References:
Selenium, an essential trace element for humans, has a direct effect on thyroid hormone metabolism and oxidation-reduction processes. The functioning of the thyroid gland is critically dependent on iodine and selenium, in order to ensure that it functions properly. An insufficient amount of selenium in the body is associated with an increased risk of thyroid disease.1
Selenium can be considered the key to the health of the thyroid gland. It is a necessary trace element for the synthesis and functioning of thyroid hormones. The concentration of selenium in the thyroid gland is higher than in any other organ in the body. Selenium works with iodine to activate three different selenium-dependent iodothyronine deiodinases, which can then activate or deactivate thyroid hormones. All three isoforms of deiodinases are selenium-containing enzymes, so dietary or supplemental selenium is essential for triiodothyronine (T3) production. This process (and selenium) is essential for normal growth, development and metabolism.2
Selenium deficiency is associated with hypothyroidism, Hashimoto's disease, an enlarged thyroid gland, thyroid cancer, and Graves' disease.
One study of 1,900 participants found a relationship between serum selenium concentrations and the size of the thyroid gland. A protective effect of selenium against enlarged thyroid gland and thyroid tissue damage was observed. In this particular study, these results were only significant for female participants.3
Another study looked at the effect of selenium on Graves' orbitopathy (when the thyroid gland produces too much thyroid hormone). The researchers compared treatment with selenium to treatment with pentoxifylline (Pentilin), an anti-inflammatory drug. The selenium treatment group reported improved quality of life and slowed the progression of Graves' orbitopathy compared to the pentoxifylline (Pentilin) treatment group.4
- For a long time, selenium was considered a toxic element. Poisoning with this element led to the development of severe anemia, bone stiffness, hair loss, and blindness. These symptoms have been observed in humans and animals in areas where the content of this element in the soil was ~1000 times greater in comparison with soils with an average amount of selenium in the other regions of the world.
- Selenium gets its name from the Greek word "selene," which means "moon." Selene was the Greek goddess of the moon.
- Selenium is a nonmetal. Like many nonmetals, it exhibits different colors and structures (allotropes) depending on the conditions.
- Brazil nuts are high in selenium, even if they are grown in soil that is not rich in the element. A single nut provides enough selenium to meet the daily requirement for a human adult.
- The primary use of selenium is to decolorize glass, to color glass red, and to make the pigment China Red. Other uses are in photocells, in laser printers and photocopiers, in steels, in semiconductors, and in assorted medicinal preparations.
- Selenium is protective against mercury poisoning.

Zinc
Zinc is one of the most important microelements of a human body. It is required for protein synthesis and production of essential hormones. Chronic zinc deficit may cause neurological and mental disorders, for example, depression. Zinc is crucial for the proper metabolism of thyroid gland hormones; zinc deficiency can cause a decrease in thyroid hormone levels and affect the rate of metabolism under a state of rest. Zinc strengthens the immune system. It plays a role in all aspects of immune function, including a crucial role in the development of T-cells (the main immune cells) and the repair of the thymus (the main organ of immune genesis).
Zinc is an essential bio-element, which plays a fundamental role in a wide range of biochemical processes. This metal is a major component of various proteins and is an important modulator of the immune and nervous systems. It is the second most abundant trace metal in humans after iron and it is the only metal which appears in all enzyme classes.
Zinc is involved in numerous aspects of cellular metabolism. It is required for the catalytic activity of over 300 enzymes and 1000 transcription factors and it plays a role in immune function, protein synthesis, wound healing, DNA synthesis and cell division. Zinc also supports normal growth and development during pregnancy, childhood, and adolescence and is required for proper sense of taste and smell.
In the brain, zinc is stored in specific synaptic vesicles by glutamatergic neurons and can modulate neuronal excitability. It plays a key role in synaptic plasticity and so in learning. Zinc homeostasis also plays a critical role in the functional regulation of the central nervous system. Dysregulation of zinc homeostasis in the central nervous system that results in excessive synaptic zinc concentrations is believed to induce neurotoxicity through mitochondrial oxidative stress (e.g., by disrupting certain enzymes involved in the electron transport chain), the dysregulation of calcium homeostasis, glutamatergic neuronal excitotoxicity, and interference with intraneuronal signal transduction.
At the same time, studies have shown a correlation between zinc deficiency and thyroid gland hormone levels. Zinc is necessary for the proper functioning of the enzyme deiodinase, which activates the inactive thyroxine (T4) hormone into a more active form - triiodothyronine (T3), ensuring all thyroid gland functions in the human body.
Zinc affects several aspects of the immune system. It is crucial for the normal development and function of innate immune cells, neutrophils and natural killer or NK cells. Zinc deficiency also affects macrophages – large cells that draw in and digest foreign particles. Zinc deficiency affects phagocytosis, intracellular killing and cytokine production. Zinc deficiency negatively affects the growth and function of T and B cells. Zinc’s ability to act as an antioxidant and stabilise membranes suggests that it plays a role in preventing free radical-induced injury during inflammatory processes.
Zinc deficiency is characterized by growth retardation, loss of appetite, and impaired immune function. In more severe cases, zinc deficiency causes hair loss, diarrhea, delayed sexual maturation, impotence, hypogonadism in males, and eye and skin lesions. Weight loss, delayed healing of wounds, taste abnormalities, and mental lethargy can also occur. Many of these symptoms are non-specific and often associated with other health conditions; therefore, a medical examination is necessary to ascertain whether a zinc deficiency is present. People at risk of zinc deficiency or inadequacy need to include good sources of zinc in their daily diets.
Groups at Risk of Zinc Inadequacy
1. People with gastrointestinal and other diseases
Gastrointestinal surgery and digestive disorders (such as ulcerative colitis, Crohn’s disease, and short bowel syndrome) can decrease zinc absorption and increase endogenous zinc losses primarily from the gastrointestinal tract and, to a lesser extent, from the kidney. Other diseases associated with zinc deficiency include malabsorption syndrome, chronic liver disease, chronic renal disease, sickle cell disease, diabetes, malignancy, and other chronic illnesses. Chronic diarrhea also leads to excessive loss of zinc.
2. Vegetarians
The bioavailability of zinc from vegetarian diets is lower than from non-vegetarian diets because vegetarians do not eat meat, which is high in bioavailable zinc and may enhance zinc absorption. In addition, vegetarians typically eat high levels of legumes and whole grains, which contain phytates that bind zinc and inhibit its absorption.
3. Pregnant and lactating women
Pregnant women, particularly those starting their pregnancy with marginal zinc status, are at increased risk of becoming zinc insufficient due, in part, to high fetal requirements for zinc. Lactation can also deplete maternal zinc stores.
4. Older infants who are exclusively breastfed
Breast milk provides sufficient zinc (2 mg/day) for the first 4–6 months of life but does not provide recommended amounts of zinc for infants aged 7–12 months, who need 3 mg/day. In addition to breast milk, infants aged 7–12 months should consume age-appropriate foods or formula containing zinc. Zinc supplementation has improved the growth rate in some children who demonstrate mild-to-moderate growth failure and who have a zinc deficiency.
5. People with sickle cell disease
Results from a large cross-sectional survey suggest that 44% of children with sickle cell disease have a low plasma zinc concentration, possibly due to increased nutrient requirements and/or poor nutritional status. Zinc deficiency also affects approximately 60%–70% of adults with sickle cell disease.
6. Alcoholics
Approximately 30%–50% of alcoholics have low zinc status because ethanol consumption decreases intestinal absorption of zinc and increases urinary zinc excretion. In addition, the variety and amount of food consumed by many alcoholics is limited, leading to inadequate zinc intake.
Zinc in the diet. Adults who are not found or established to be deficient in Zinc are normally required 11 mg for men, 8 mg for women and 11 mg for pregnant women of Zinc per day
Zinc supplements have the potential to interact with several types of medications. A few examples are provided below. Individuals taking these medications on a regular basis should discuss their zinc intakes with their healthcare providers.
1. Antibiotics
Both quinolone antibiotics (such as Cipro®) and tetracycline antibiotics (such as Achromycin® and Sumycin®) interact with zinc in the gastrointestinal tract, inhibiting the absorption of both zinc and the antibiotic. Taking the antibiotic at least 2 hours before or 4–6 hours after taking a zinc supplement minimizes this interaction.
2. Penicillamine
Zinc can reduce the absorption and action of penicillamine, a drug used to treat rheumatoid arthritis. To minimize this interaction, individuals should take zinc supplements at least 2 hours before or after taking penicillamine.
3. Diuretics
Thiazide diuretics such as chlorthalidone (Hygroton®) and hydrochlorothiazide (Esidrix® and HydroDIURIL®) increase urinary zinc excretion by as much as 60%. Prolonged use of thiazide diuretics could deplete zinc tissue levels, so clinicians should monitor zinc status in patients taking these medications.
Zinc is found in abundance in the human brain. Patients with depression may have decreased consumption of food sources rich in zinc and consistently low dietary zinc intakes may contribute to depressive symptoms by further lowering available zinc, therefore zinc supplementation may have a potential influence on depressive symptoms. In preclinical studies, single- or long- term treatment with zinc has been shown to have antidepressant-like effects.
According to recently introduced hypotheses of antidepressant action, one of major goals to be modified by an antidepressant is the NMDA glutamate receptor. The mechanism of antidepressant activity of zinc might be related to its direct antagonism at NMDA receptor. Besides the central nervous system, zinc is also involved in the immune/inflammatory regulation in depressive disorders.
In the hippocampus and cortex, zinc ions regulate synaptic transmission or act as neurotransmitters, modulating many ligand- and voltage-gated ion channels. Disruption of zinc homeostasis in these regions has been implicated in many disturbances in cognition, behavioral and emotional regulation through mechanisms of decreased neurogenesis and neuronal plasticity.
Zinc deficiency has also been implicated in the endocrine pathway of depression. Persistently high levels of cortisol have been implicated in the development of depression via hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis. Increased plasma cortisol levels could, therefore, potentially mediate the relationship between zinc deficiency and depression.
The relationship between serum zinc levels and depression could be partially explained by reverse causation, whereby depression influences the intake, bioavailability or biological regulation of zinc. Oxidative stress and its accompanying immune-inflammatory response have been linked to the pathophysiology of depression. In response to oxidative stress, levels of pro-inflammatory cytokines (e.g., interleukin 1 (IL-1) and IL-6) increase and, in turn, decrease of the level of albumin and increase the synthesis of metallothioneins. Albumin is the main zinc transporter, and a decrease in albumin coupled with an increase in metallothioneins may compound to decrease serum levels of zinc.
References
Zinc is an essential element involved in many basic biochemical reactions of the thyroid gland. Zinc is necessary for the production of the hormones triiodothyronine (T3), thyroxine (T4) and thyroid-stimulating hormone (TSH).
T4 is the main thyroid gland hormone, which is a kind of bodyreserve, while T3 is a much more active hormone. If necessary, one molecule of iodine is separated from T4, and it turns into an active hormone - T3.
This trace element participates in the synthesis of thyrotropin-releasing hormone (TRH) in the hypothalamus and in the synthesis of thyrotropin or thyroid-stimulating hormone (TSH) in the pituitary gland.1
Some studies have shown that zinc deficiency and low zinc concentration in the serum can cause changes in thyroid gland structure and thyroid gland hormone metabolism. Also, studies have shown that taking zinc can increase the concentration of thyroid gland hormones.1
In a study where subjects took zinc supplements, they had improvements in all thyroid gland hormone levels (especially T3) as well as in the rate of metabolism in the state of rest. Another study showed that taking zinc alone or in combination with selenium can improve thyroid gland function in women with hypothyroidism.2
Zinc deficiency can cause hypothyroidism. On the other hand, hypothyroidism can cause zinc deficiency because thyroid gland hormones are needed for zinc absorption.
Zinc has been found to be an essential trace element for the immune system. However, at the cellular and molecular level, the mechanisms of zinc actions on the immune system are relatively recent and its effects are diverse.
Adequate levels of zinc in the body are essential for the formation and function of different populations of lymphocytes (the main immune cells), such as the division, maturation and differentiation (development into different forms) of T-cells (or T-lymphocytes); lymphocyte response to mitogens (small bioactive proteins or peptides that induce cells to start dividing or increase the rate of division). At the same time, zinc is important for programming lymphoid and myeloid cell death; gene transcription and biomembrane function. Lymphocytes are one of the types of cells activated by zinc. Zinc is a structural component of various proteins, neuropeptides, hormone receptors and polynucleotides. Zinc deficiency results in rapid and marked atrophy of the thymus, impaired cell-mediated cutaneous sensitivity and lymphopenia. Primary and secondary antibody responses are reduced in zinc deficiency, particularly for those antigens that require T-cell help, such as those in heterologous red blood cells. In addition, antibody response and the generation of splenic cytotoxic T-cells after immunisation are reduced. Zinc also inhibits the production of tumour necrosis factor, which is implicated in the pathophysiology of cachexia and wasting in acquired immune deficiency syndrome.
In short, zinc is absolutely essential for the functioning of the thymus and the normal functioning of the immune system. Zinc prevents immunodeficiency by stimulating antibody synthesis and providing antiviral effects.
- The element was probably named by the alchemist Paracelsus after the German word Zinke and supposedly meant "tooth-like, pointed or jagged" (metallic zinc crystals have a needle-like appearance). Zink could also imply "tin-like" because of its relation to German zinn meaning tin.
- The oldest known pills were made of the zinc carbonates hydrozincite and smithsonite. The pills were used for sore eyes and were found aboard the Roman ship Relitto del Pozzino, wrecked in 140 BC.
- Alchemists burned zinc metal in air and collected the resulting zinc oxide on a condenser. Some alchemists called this zinc oxide lana philosophica, Latin for "philosopher's wool", because it collected in wooly tufts, whereas others thought it looked like white snow and named it nix album.
- Zinc is a natural insect repellent and sun screen, protecting lips and skin.
- Zinc is 100% recyclable. Over 80% of the zinc available for recycling is currently recycled.

Calcium
Calcium helps build bones and teeth and is essential for nerve, enzyme, heart, muscle and blood clotting functions. Insufficient intake of this mineral can cause bone weakness and increase the risk of fractures in the elderly. Calcium is a natural sleep aid that can help you fall asleep and ensure peaceful sleep because calcium helps the brain use the amino acid tryptophan to produce the natural sleep-inducing hormone melatonin, which helps you fall asleep and stay asleep.
Calcium is the mineral that is mostly associated with healthy bones and teeth, although it also plays an important role in blood clotting, helping muscles to contract. At the same time, calcium regulates a normal heart rhythm and ensures nerve functions, as well as participates in the regulation of hormone activity, reduces neuromuscular excitement, participates in the absorption and use of vitamin B12. About 99% of the calcium in the body is in the bones, with the remaining 1% in the blood, muscles and other tissues.
There are three forms of calcium in the blood plasma: 41% is bound to proteins and in this way it cannot cross the capillary membrane; 9% is in combination with anions and interstitial (tissue) fluid, able to cross the capillary membrane and 50% is ionised and able to cross the capillary membrane. Calcium plays an important role in cellular and extracellular fluid exchange. Calcium ions are necessary in the transmission of nerve impulses.
In neurons, calcium is a key element and it performs multiple tasks. It helps spread electrical signals along axons (nerve cell projections conducting nerve impulses to other nerve cells). It activates synaptic connections to carry neurotransmitters (transmit nerve impulse from synapse to cell) into synapses. Calcium is also involved in memory formation, metabolism and cell growth.
All cells from primitive unicellular organisms to highly differentiated neurons in the cerebral cortex depend on calcium metabolism. This element is important in living organisms, especially in cell physiology, where the movement of Ca2+ in and out of the cytoplasm acts as a signal for many cellular processes.
In food supplements, calcium is found in various forms, where each of the compounds contains a different amount of calcium, or the basic substance, elemental calcium. The following forms of calcium are most commonly used in food supplements:
- • Calcium carbonate (40% elemental calcium)
- • Calcium citrate (21% elemental calcium)
- • Calcium lactate (13% elemental calcium)
- • Calcium gluconate (9% elemental calcium)
Calcium carbonate contains significantly more elemental calcium than other compounds, but requires an acidic food or drink to absorb it. The acid in calcium citrate, on the other hand, promotes the absorption of the compound in an environment of reduced acidity and may be more effective in people taking antacids.
Long-term calcium deficiency can cause changes in teeth (teeth can suddenly become more sensitive, softer and more easily injured; it may be the case that the tooth simply breaks when biting on harder food), cataracts, changes in the brain and osteoporosis, which causes brittle bones.
Symptoms of calcium deficiency:
A person with calcium deficiency may experience:
- • muscle pain, cramps and spasms,
- • pain in the thighs and arms when walking or moving,
- • numbness and tingling in the arms, legs and feet and around the mouth.
These symptoms may come and go, but they do not, however, tend to disappear with activity. More extreme sensations may indicate a more severe deficiency, which can lead to: seizures, arrhythmia, and even death.
Low calcium levels can cause extreme fatigue, which includes a lack of energy and a general feeling of sluggishness. It can also cause insomnia. Fatigue associated with calcium deficiency can also include dizziness and double vision, characterised by a lack of focus, oversight, and confusion.
Prolonged calcium deficiency can cause:
- • dry skin
- • dry, broken or brittle nails
- • brittle hair
- • alopecia, which causes hair loss in the form of spots
- • eczema or skin inflammation that can cause itchy or dry spots
- • psoriasis
Bones store calcium well, but they need high levels of calcium to be strong. If total calcium levels are low, the body can divert some ofthe calcium from the bones to processes needed by the body, making them brittle and prone to fracture.
Over time, too little calcium can cause osteopenia, a decrease in bone mineral density. This can lead to osteoporosis, which makes the bones thinner and more vulnerable to fractures, as well as pain and postural problems.
Some studies suggest that calcium deficiency may be associated with mood disorders, including depression.
Anyone who suspects that calcium deficiency is contributing to depressive symptoms should consult a doctor. After checking your calcium levels, your doctor may recommend additional calcium intake.
For the body to use calcium, it is worth knowing some nuances:
- • together with calcium, vitamin D is needed - both help each other fulfil their functions;
- • in order for calcium to strengthen bones and teeth, sufficient phosphorus must be taken;
- • appropriate enzymes are needed in the gastrointestinal tract, which dissolve the ingested calcium;
- • excessive use of coffee or salt promotes the release of calcium from the body;
- • bone robbers include oxalic acid present in certain vegetables such as rhubarb and spinach;
- • cocoa and black tea negatively affect the absorption of calcium in the body;
- • a large amount of sugar, salt, phosphates and fat in the diet has a negative effect on calcium absorption. Fast snacks, ready-made meals, meat and sausage products contain a particularly large amount of phosphates, so their consumption should be moderate;
- • absorption is hindered by fatty and greasy food, white bread, wheat bran. Absorption can be enhanced by vitamin C and products containing it;
- • any disease of a gastroenterological nature reduces the ability to absorb calcium.
Some foods that are rich in calcium: dairy products such as milk, cheese and yoghurt; beans; figs; broccoli; tofu; soy milk and spinach.
Adults who have not been observed to have a calcium deficiency need 1,000 mg of calcium per day, persons over 51 years of age - 1,200 mg per day.
There is an inverse relationship between calcium intake and absorption. Calcium absorption from food is about 45% when 200 mg/day is taken, but only 15% when intake exceeds 2,000 mg/day. Age can also affect the absorption of calcium taken in from food. Dietary absorption of calcium is as high as 60% in infants and young children, who need significant amounts to build bone, but this drops to about 25% in adulthood and continues to decline with age.
Bloating, gases and constipation may be observed when taking calcium supplements. Very high doses of calcium can cause kidney stones.
Interaction. If you regularly take any prescription or over-the-counter medications, ask your doctor if it is safe to take extra calcium supplements. Calcium can interact with medications for treating heart disease, diabetes, epilepsy, and other diseases. High doses of vitamin D can lead to dangerously high calcium levels. High doses of calcium can also prevent your body from absorbing minerals such as iron and zinc. Try to take calcium one to two hours before or after taking other food supplements or medications. If you happen to take calcium at the same time as other medications or food supplements, they may interact with these products and they will be excreted from your body without being absorbed.
Calcium supplements can interact or interfere with certain medications, and some medications can lower calcium levels in the body. Here are some examples:
Risks. If you have kidney disease, heart problems, sarcoidosis, or bone tumours, do not take calcium supplements unless directed by your doctor.
Overdose. High levels of calcium in the blood can cause nausea, dry mouth, stomach pain, irregular heartbeat, confusion and even death.
Calcium is directly related to our sleep cycles. In one study published in the European Neurology Journal, researchers found that calcium levels in the body are higher during some of the deeper stages of sleep, such as the rapid eye movement (REM) phase. The study concluded that sleep disorders, specifically a lack of REM deep sleep or disturbed REM sleep, are associated with calcium deficiency. Normal sleep patterns were restored after blood calcium levels normalised.
Calcium helps the brain to use the amino acid tryptophan to produce the sleep-inducing substance melatonin.
If the body lacks calcium, nerve impulses may be inhibited and unstable, leading to excessive anxiety or stress. In addition, the nervous system will encounter many obstacles in its operation: the contraction of the heart will be disturbed and the function of muscle reflexes will change. If calcium deficiency is prolonged, insomnia, difficulty falling asleep, poor sleep and frequent awakening will be observed.
Calcium deficiency can cause other related diseases, such as peptic ulcers, which also seriously affect sleep. Lack of calcium stimulates the increase of stomach acid; long-term increased acid will cause damage to the stomach lining, and even ulcers. This process is often accompanied by symptoms such as heartburn, nausea and nighttime stress that causes insomnia.
At the same time, it should be mentioned that there is a close relationship between vitamin D levels and calcium levels, where calcium levels regulate the formation of the deep sleep phase, while the classical functions of vitamin D include intestinal calcium transport and bone mineralisation, which are essential for calcium homeostasis. It is possible that sleep disorders in the case of vitamin D deficiency may be related to altered calcium levels. Lower calcium level in the serum may be associated with more impaired sleep-wake control and rest-activity rhythms. [[2:: Atsauce: Yi-Seon Jeon, Seungyeong Yu, Chaeyeon Kim, Hyuk Joo Lee, In-Young Yoon and Tae Kim1 “Lower Serum Calcium Levels Associated with Disrupted Sleep and Rest–Activity Rhythm in Shift Workers”; Nutrients. 2022 Aug; 14(15): 3021.; Published online 2022 Jul 22. doi: 10.3390/nu14153021]]
 Free delivery to Omniva parcels throughout the Baltics for purchases from 20 euros!
Free delivery to Omniva parcels throughout the Baltics for purchases from 20 euros!